Draw the electron configuration for a neutral atom of titanium. – Embark on an exploration into the electron configuration of a neutral titanium atom, a journey that unlocks the secrets of this versatile element. As we delve into its orbital structure and delve into the intricacies of its chemical behavior, a deeper understanding of titanium’s remarkable properties awaits.
Titanium, a transition metal nestled within Group 4 of the periodic table, stands out for its exceptional strength, corrosion resistance, and lightweight nature. Its electron configuration plays a pivotal role in shaping these remarkable attributes, influencing its chemical reactivity and defining its position within the periodic landscape.
Electron Configuration of Titanium: Draw The Electron Configuration For A Neutral Atom Of Titanium.
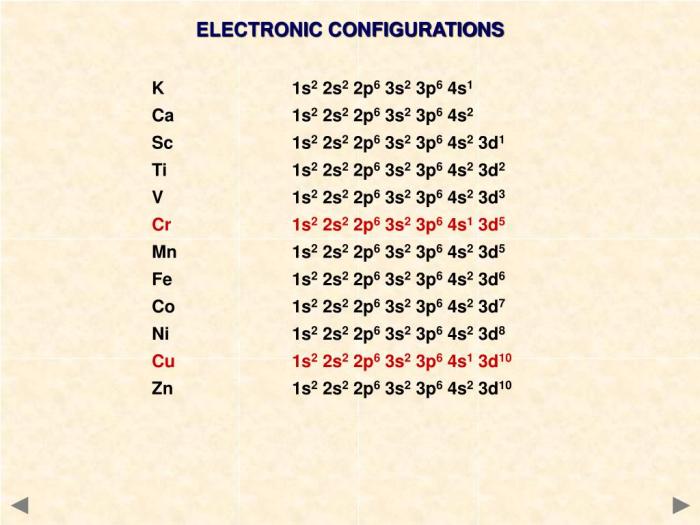
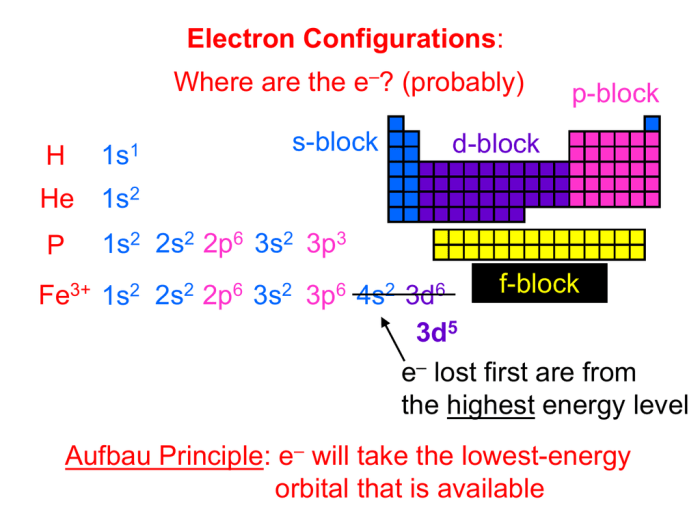
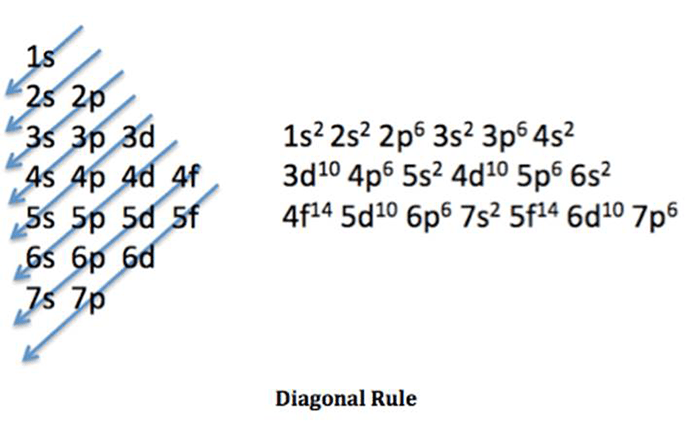
The electron configuration of an element describes the arrangement of electrons in the atomic orbitals around the nucleus. It plays a crucial role in understanding the chemical properties and behavior of an element.
The electron configuration of a neutral atom of titanium (Ti) is:
s22s 22p 63s 23p 63d 24s 2
Orbital Diagram of Titanium
An orbital diagram is a visual representation of the electron configuration. It shows the distribution of electrons in the atomic orbitals:

- 1s: 2 electrons
- 2s: 2 electrons
- 2p: 6 electrons
- 3s: 2 electrons
- 3p: 6 electrons
- 3d: 2 electrons
- 4s: 2 electrons
Valence Electrons of Titanium, Draw the electron configuration for a neutral atom of titanium.
Valence electrons are the electrons in the outermost energy level of an atom. Titanium has two valence electrons in the 4s orbital.
Valence electrons are responsible for chemical bonding. Titanium’s two valence electrons allow it to form bonds with other atoms, such as oxygen and nitrogen.
Electron Configuration and Periodic Table
The electron configuration of titanium helps determine its position in the periodic table. Titanium is in Group 4 (previously known as Group IVB) and Period 4.
Elements in the same group have the same number of valence electrons. Titanium’s two valence electrons place it in Group 4.
Elements in the same period have the same number of electron shells. Titanium’s four electron shells place it in Period 4.
Detailed FAQs
What is the electron configuration of a neutral titanium atom?
The electron configuration of a neutral titanium atom is [Ar]3d 24s 2, indicating that it has two electrons in the 3d subshell and two electrons in the 4s subshell.
How does the electron configuration influence the chemical properties of titanium?
The electron configuration determines the number of valence electrons, which are responsible for chemical bonding. Titanium’s two valence electrons allow it to form diverse chemical bonds, contributing to its versatility and reactivity.
Why is titanium known for its exceptional strength and corrosion resistance?
Titanium’s strength and corrosion resistance stem from its strong metallic bonds and the formation of a protective oxide layer on its surface. The electron configuration plays a role in stabilizing these bonds and promoting the formation of the protective oxide layer.